Rapid Antibody Test OraQuick® HCV Immunoassay Hepatitis C Test CLIA Waived 25 Tests, 25/KT
$101.00
Description
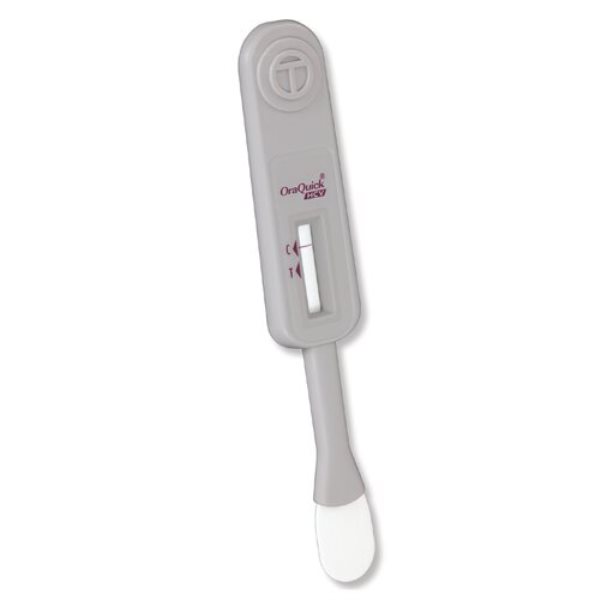
<ul><li>The OraQuick® HCV test is FDA approved for detecting HCV antibodies in fingerstick and venipuncture whole blood</li><li>Our simple platform enables healthcare providers to deliver an accurate diagnosis in 20 minutes</li><li>The OraQuick HCV Rapid Antibody Test is comprised of both a single-use test device and vial containing a pre-measured amount of a buffered developer solution</li><li>The test consists of a sealed pouch with two separate compartments for each component</li><li>The developer solution facilitates the capillary flow of the specimen into the device and onto the assay strip</li><li>Kit includes: 25 divided pouches each containing a test device, absorbent packet, and developer solution vial (each contains 0.75 mL of a buffered saline solution with an antimicrobial agent); 5 reusable test stands; 25 specimen collection loops; package insert</li></ul>
Be the first to review “Rapid Antibody Test OraQuick® HCV Immunoassay Hepatitis C Test CLIA Waived 25 Tests, 25/KT” Cancel reply
You must be logged in to post a review.
Reviews
There are no reviews yet.