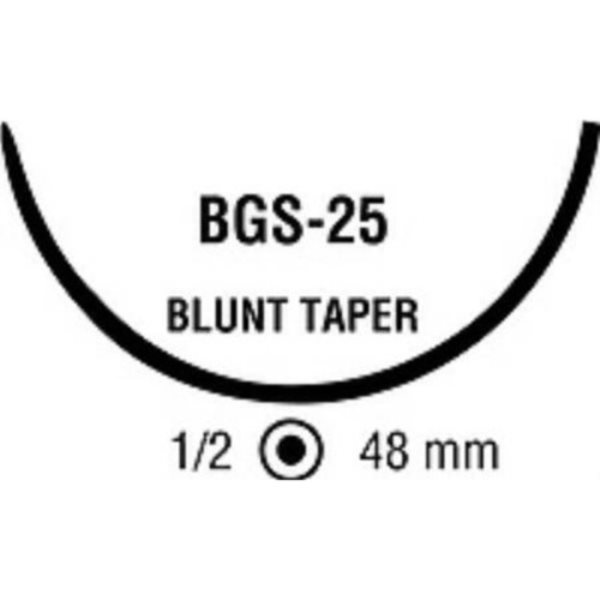
suture with needle Maxon Absorbable Uncoated Green Suture Monofilament Polyglyconate Size 0 36 Inch Suture 1-Needle Size 0 1/2 Circle Blunt Taper Point Needle, 12/BX
$67.03
Description
<ul><li>MAXON™ and MAXON™ CV synthetic absorbable sutures are prepared from polyglyconate, a copolymer of glycolic acid and trimethylene carbonate</li><li>MAXON™ and MAXON™ CV synthetic absorbable sutures are indicated for use in general soft tissue approximation and/or ligation, including use in pediatric cardiovascular tissue, where growth is expected to occur, and in peripheral vascular surgery</li><li>MAXON™ and MAXON™ CV synthetic absorbable sutures are not indicated for use in adult cardiovascular tissue, ophthalmic surgery, microsurgery and neural tissues</li><li>MAXON™ sutures retain approximately 75% of initial tensile strength at two weeks, 65% of at three weeks and 50% at four weeks post-implant</li><li>Absorption of MAXON™ sutures is minimal until about 60 days after implant and is essentially complete within six months</li><li>This product is required to be reported under California Proposition 65</li></ul>
Be the first to review “suture with needle Maxon Absorbable Uncoated Green Suture Monofilament Polyglyconate Size 0 36 Inch Suture 1-Needle Size 0 1/2 Circle Blunt Taper Point Needle, 12/BX” Cancel reply
You must be logged in to post a review.
Reviews
There are no reviews yet.